Discovery of Island cells which control connection of two events separated in time into a single experience
-A big step toward elucidation for the control mechanism of temporal association memory-
Dr. Takashi Kitamura and his colleagues at RIKEN-MIT Center for Neural Circuit Genetics (BSI-MIT) lead by Dr. Susumu Tonegawa, MIT Professor and Director of RIKEN BSI in collaboration with Dr. Kuniya Abe, Team Leader of Technology and Development Team for Mammalian Cellular Dynamics and Dr. Atsushi Yoshiki, Head of Experimental Animal Division, RIKEN BRC have discovered Island cells and a new brain circuit which control memory formation of connecting two events separated in time into a single experience in the journal of Science published on line on January 23, 2014.
Dr. Yuichi Obata, Director of RIKEN BRC and Dr. Nathan Mise*, Research & Development Scientist of Technology and Development Team for Mammalian Cellular Dynamics, RIKEN BRC have also participated in this collaboration and supported this study.
*present affiliation: Jichi Medical University
Please see RIKEN Press Release on January 24, 2014 for more about this story.
| Islands in the brain: new circuit shapes memory formation | |
|---|---|
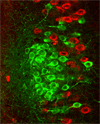 |
RIKEN and MIT scientists have discovered Island cells and a new brain circuit responsible for limiting the formation of temporal association memory in the entorhinal cortex-hippocampus network. Read more... |
Researchers at BRC have accumulated expertise in recombinant DNA technologies using bacterial artificial chromosome (BAC), generation of BAC transgenic mice and histological analysis of tissue specific expression of Cre recombinase. With such expertise, BRC contributed in this study to generate and histologically analyze Wfs1-Cre mice which expressed Cre recombinase specifically in the Island cells in the entorhinal cortex.
Generation and analysis of Wfs1-Cre mice
During the period from 2008 to 2012, BRC had collaborated with BSI-MIT, developed brain sub-region specific Cre mice of 128 lines with 39 promoter genes by using BAC recombineering and conducted the histological analysis of site specific Cre expression for neural circuit genetics studies.
Researchers at BSI-MIT discovered that the Wfs1 gene was specifically expressed in Island cells in the entorhinal cortex layer II (ECII), but not in the ECII Ocean cells.
Then, we jointly developed and analyzed Wfs1-Cre mice which specifically expressed Cre recombinase in the ECII Island cells. By injecting the Cre-dependent adeno-associated virus vector for expression of fluorescence and optogenetics proteins into the superficial layer of the entorhinal cortex of the Wfs1-Cre mice, we could successfully label, activate and suppress the Island cells specifically.
A series of experiments revealed that the newly identified Island cells sent their bundled projections to the CA1 region of the hippocampus and connected to the stratum-lacunosum interneurons that suppressed the memory-making neurons in CA1.
Thus, brain sub-region specific Cre mice in combination with other advanced technologies are expected to play a pivotal role for elucidation of accurate structures and functions of neural circuits in the brain.
Publication
Kitamura T, Pignatelli M, Suh H, Kohara K, Yoshiki A, Abe K, Tonegawa S.
Island Cells Control Temporal Association Memory
Science, 2014 Feb 21, 343: 896-901, doi; 10.1126/science.1244634